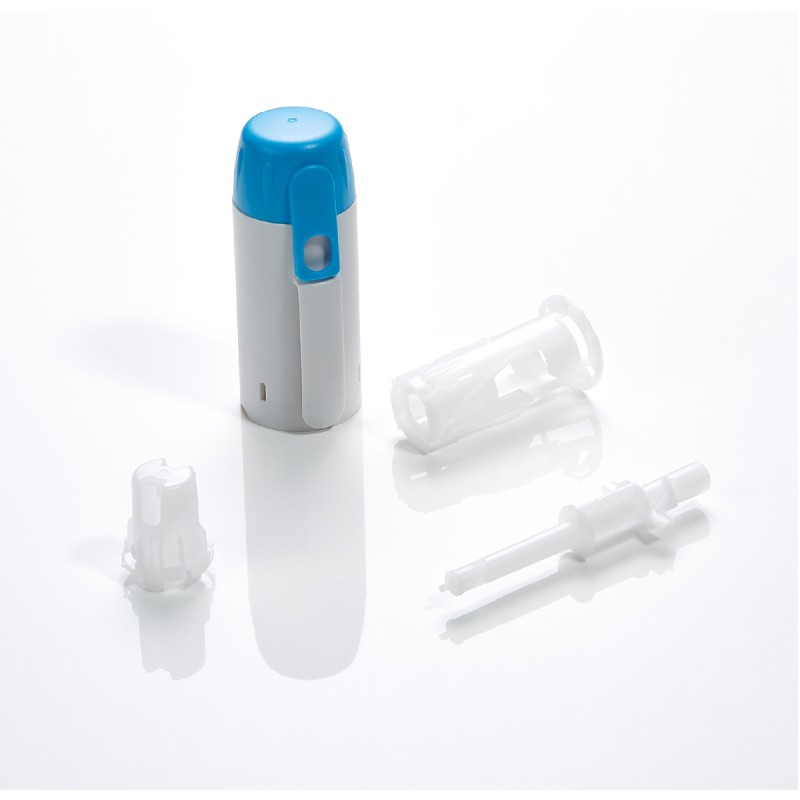
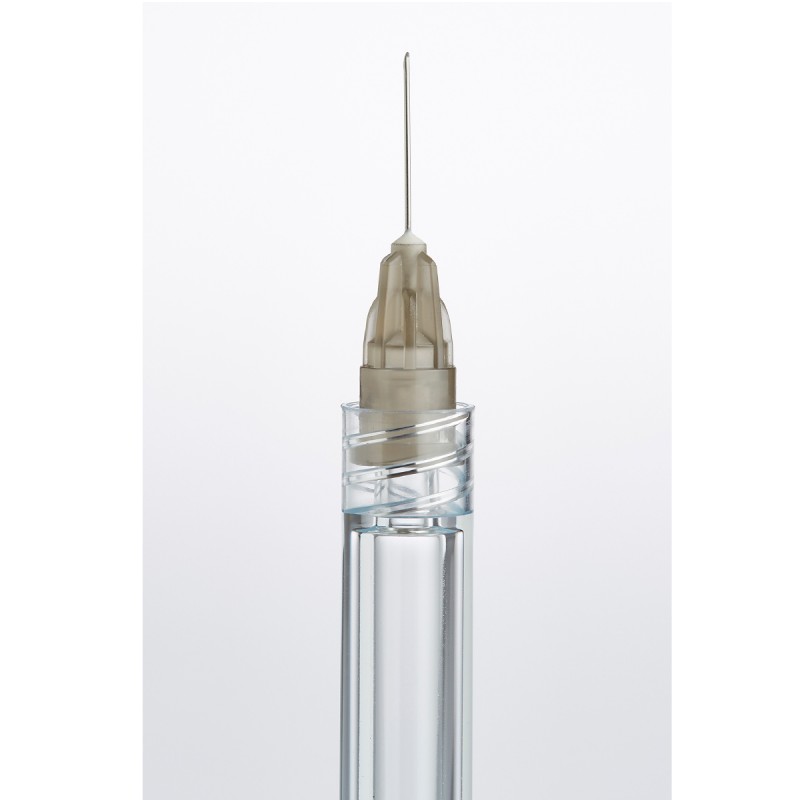
CLAYENS NP is the chosen partner of recognised players in the health industry. Whatever the business sector (pharmaceutical companies, diagnostic laboratories, medical device manufacturers, etc.) and size (multinational or innovative start-up) of our clients, we support and secure the development of their medical devices and pharmaceutical packaging.
Our recognised know-how in plastic injection and plastic machining enables us to offer our discerning clients comprehensive and customised support, from the design of their devices through to production, in accordance with international regulatory requirements (ISO 13485-2016, ISO 15378 and QSR - 21 CFR part 820). .
Clayens NP Healthcare Services
R&D

Expertise
• Modelling / Design concept
• Product design and development (CAD, rheology, drawings, etc.)
• Price optimisation / Process automation / Controls and laboratory tests
Infrastructure / facilities
• Rapid prototyping and 3D printing
• R&D laboratory: functional and dimensional validation
• Pilot line (ISO7) for the production of clinical batches
Technologies

• Injection molding
• 2K injection
• Overmoulding
• Plastic machining
• Thermoforming
• Additive manufacturing

• Assembly
• Marking
• Control
• Packaging
• Sterilisation (subcontracted)
Qualification

Process validation :
• FOT (first of tool)
• SAT (Site Acceptance test)
• IQ (Installation Qualification)
• OQ (Operational Qualification)
• PQ (Operational Qualification)
Product validation :
• Functional product validation
• Shelf life validation
• Transport validation
• Biocompatibility (subcontracted)

Regulatory Affairs :
• Regulatory watch
• Support for technical documentation and CE registration
• Support for FDA registration
Production

Manufacturing in a controlled environment (ISO7)
• 5 ISO7 clean rooms
• 129 injection moulding machines for health (from 25 to 320 tons)
• 2, 3 and 5-axis machining
Production available in very small series/very large series
Global footprint (4 plants dedicated to health in 3 different countries)
Our health certifications




Our markets segments :
Medical devices
Thanks to our expertise, we are involved in developing and manufacturing customised medical devices in categories I, II and III :
Surgical kits (orthopaedics, dentistry, ophthalmology, etc.)
We are pioneers in the design and development of specific and customised single-use surgical kits.
Our kits are used in spinal surgery, for example, to repair fractures.
We also use overmoulding technology to manufacture a wide range of ancillary instruments for implant placement or removal, in particular suture anchors.
Implant positioning systems (ophthalmology, aesthetics, orthopaedics, etc.)
Our experience in solid or viscous implant delivery systems is widely recognised, particularly in ophthalmology (intraocular implant injectors for cataract operations, IOL injector sets for glaucoma), aesthetics (extra-long 1 ml syringe for injection of hyaluronic acid for wrinkle filling) and orthopaedics (bone substitute injectors and suture anchor delivery systems).
Hospital kits (dialysis, acute care, etc.)
Our offer extends to specific hospital kits and systems, designed in collaboration with medical device companies for numerous applications, including parts used in dialysis, rib cage drainage systems, epidural catheters, filtration systems and catheter connectors.
Implants (orthopaedics)
We manufacture suture anchors for major orthopaedic companies to fix tendons and ligaments to the bone. We also make PEEK implants for orthopaedic and dental applications, using injection and machining technologies in the manufacturing process.
Implants packaging (orthopaedic and dental)
We manufacture implant and instrument packaging systems for the dental and orthopaedic fields. Used for the sterilisation of implants and ancillary instruments, these systems can be made of plastic (Radel), stainless steel or hybrids (plastic and stainless steel). AIP Médical's standard range can be customised in accordance with our clients’ requirements.
Pharmaceutical packaging
CLAYENS NP's Healthcare division is a leading pharmaceutical packaging producer thanks to the performance of its manufacturing facilities and the trust of major pharmaceutical partners. Our expertise is currently benefiting a wide range of pharmaceutical and biotechnological applications.
Injectable drug delivery systems (primary packaging)
Leading pharmaceutical companies request our expertise for the manufacture of injectable drug delivery systems. In particular, we manufacture auto-injectors (for the injection of viscous drugs, as well as for the injection of emergency treatments). Our know-how also extends to primary packaging. We are able to meet the most stringent requirements for injectable drug delivery systems, as demonstrated by our ready-to-fill COC syringe.
Secondary packaging
Thanks to our expertise in pharmaceutical packaging and our production capacity, we manufacture syringe packaging components known as Tubs (packaging containers) and Nests (packaging trays) at several of our sites (in Mexico, France and Poland).
Drug delivery system components
CLAYENS NP’s Healthcare Division has been successfully involved in the manufacture of components for drug delivery systems for many years. Our products include spray system components, such as pumps, sprays, special nozzles and inhalers. We also manufacture safety systems for syringes (caps), finger rests and plunger rods.
Diagnostics
For more than thirty years, the Health division of CLAYENS NP has been involved in the design, industrialisation and manufacture of in vitro diagnostic kits. The skills of our teams and the performance of our facilities have earned the trust of our main in vitro diagnostic partners. Our company, which is a leader in the outsourcing of In Vitro Diagnostic Kit manufacture, produces a wide range of diagnostic applications.

Cell culture kits (microbiology)
Thanks to our established know-how, we can produce culture kits, including petri dishes, using rapid and just-in-time production methods. Our industrial expertise - double-stage moulds, high-speed automation processes and moulding of transparent consumables - meets even the most exacting requirements.
Cell culture kits (microbiology)
Our offer extends to other specific and customised consumables for diagnostics, which are used for a wide range of applications. They are the result of a fruitful working relationship with diagnostic companies, whether world leaders or start-ups.
Hygiene & Safety
For more than thirty years, the Health Division of CLAYENS NP has been involved in the design, industrialisation and manufacture of hygiene and safety products. The trust major partners place in us and the performance of our production resources place us in a leading position for the outsourcing of the manufacture of a wide range of safety and hygiene applications.

Needle and syringe collectors
Thanks to our expertise in high-speed automation processes and our experience in moulding, CLAYENS NP's Healthcare division is able to handle a wide range of requests in this sector, including for needle collectors required for infectious clinical waste.

Contamination-free transfer system
Our technical expertise and state-of-the-art ISO7 facilities play a key role in the success of our critical products. We manufacture parts used in clean or even aseptic transfer that meet the highest requirements for cleanliness and particulate levels.